2023中国海外人才创新创业大赛分赛区优秀项目推介 (四)
2023-08-24 14:50:36 浏览数量:次
目前,2023中国海外人才创新创业大赛分赛区比赛正在陆续进行,为更加全面、更加立体、更加详细介绍优秀参赛项目,海智计划公众号近期将持续推介分赛区优秀项目,敬请关注!
海外分赛第四赛区(新加坡)于2023年7月22日在新加坡成功举办!本期将推出第四赛区两个优秀项目信息,一起来看看吧!
一、NOUSQ:中耳积液性中耳炎手持治疗仪
项目情况
Briefly introduce the general situation of the technology or project, including the background of technology R&D, application segments, technology forms, application fields, what problems to solve, the advantages of technology, stages of development, etc.; In addition, it can score extra points if it has won awards, the core member of the team has influence (about 500 words, it would be better if there are pictures)
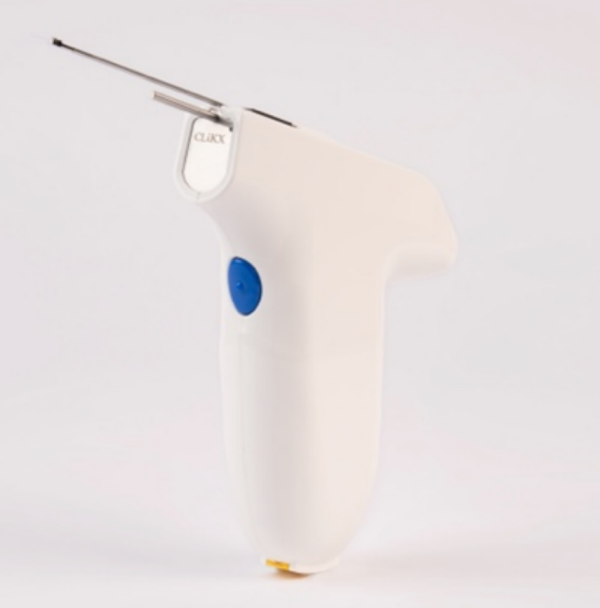
NousQ's CLiKX is the world’s first handheld robotic device with sensors to treat Otitis Media with Effusion (OME). OME is the condition where fluid is trapped behind the ear drum in the middle ear space chronically. It is the number one reason for a child’s hearing loss, visit to the doctors, use of antibiotics and general anesthesia (GA). Untreated, there is speech, language and learning delay; brain infection, reduced work and social option and great caregiver burden. When medications fail, surgery is required to place a tiny ear tube onto the ear drum. The current surgery requires many different instruments, an operating theatre, GA and an expensive non- portable surgical microscope. CLiKX can move the surgery out of the operating theatre into the clinic, as it does away with GA and the microscope. This drastically reduces treatment delay, cost, risk and inequity. Currently, only 18 million children can get to the surgery. With CLiKX, a 120 million children can be helped.
CLiKX places the ear tube in 1 second, in the clinic with just 1 click of the device, with just local anesthesia (LA). CLiKX mimics the current OME surgery performed by the ENT surgeon. Unlike current surgery, CLiKX does not need multiple surgical instruments, an operating theatre (OT), GA or the surgical microscope. CLiKX - with the help of sensors, software algorithms and robotics - performs the surgery with an atraumatic slit and weaves the ear tube on the ear drum in just 1 second. With CLiKX the entire OME procedure can be done in an outpatient clinic under local anesthesia ear drops and the patient home in an hour.
Unlike CLiKX, our competitors in US and EU are all manual devices without sensor guidance nor software for automation. The competitor devices mode of action punctures a hole in the ear drum so that their proprietary tubes tubes can be inserted through this hole. On the other hand, CLiKX mimics the current surgery by making a small slit incision on the ear drum and inserting off-the shelf proven tubes.
CLiKX is currently in first in man clinical trial at the National University Hospital in Singapore and so far has shown safety and feasibility for tympanostomy tube insertion in humans. These trials help prepare us for the US FDA clinical trials starting in Q1 2024.
We have won numerous awards such as Winner of Medtronic APAC Innovation Challenge 2022, Grand prize winner of Medtech Innovator APAC Challenge 2022, Winner Victoria Health Week Medtech Festival 2022, 2nd Prize at 2023 “Win in Suzhou, Win the Future” Venture Fest, Singapore division.
技术创新性和先进性
With the S$5 million grant received from the National Research Foundation, a functioning prototype of CLiKX was developed in Jun 2021. We were able to conduct bench and cadaver tests with the prototype with 100% success rates. In 2022, CLiKX commenced its FIM human trials at Singapore’s National University Hospital. The ongoing trials are 100% successful so far. The primary aim of the trials was to ensure safety, viability and ease of use of CLiKX. CLiKX is the world's first and only handheld automated robotic tympanostomy tube applicator. Unlike CLiKX, the other 3 devices in US and EU are all manual devices without sensor guidance nor software for automation. The competitor devices mode of action punctures a hole in the ear drum so that their proprietary tubes tubes can be inserted through this hole. On the other hand, CLiKX mimics the current surgery by making a small slit incision on the ear drum and inserting off-the shelf proven tubes.
技术目前发展水平
CLiKX is currently undergoing design freeze. It will then be transferred to pilot manufacturing, ready for pivotal clinical trials in US and Australia.
技术优势的可持续性和不可替代性
Our Razor & Blade business model is a key advantage of CLiKX technology. The single-use disposable shaft is our main revenue driver given its high margin, high sale volume potential. The main body of CLIKX is reusable (>5,000 times used) and could be sold at a nominal price subject to minimum order quantities of the single-use shaft. The CLiKX distribution model will begin initially with our own sales force as we engage the various key opinion leaders, family doctors and paediatricians directly. It is critical that we engage and establish these early contacts and relationships ourselves in the initial stage to ensure CLiKX is properly introduced to the right key opinion influencers and establishments. This will then transition into a hybrid model where the CLiKX sales force will work with reputable ENT medical device distributors to accelerate the distribution of CLiKX to the wider market. We have put in place IP protection strategies to prevent re-use and copy of CLiKX device. Our 2 main patents are also filed and granted in China.
Clinical trial results in US and Australia pose the biggest risk to the technology. This risk is mitigated by the current First-in-Man (FIM) human trials that are conducted at the National University Hospital. Initial data has shown the device to be safe (safety features have proven to be working as intended and subjects experienced no adverse events) and the grommet tubes have been inserted on the tympanic membrane without issues. The device has also been carefully optimized and tested in several bench models for over 300 times with an overall 100% success rate for grommet tube placement. Tests have also been performed on 13 cadaveric ear specimens with an overall 100% deployment of grommet tubes, showing positive outcome of the CLiKX device. In addition, early reliability test has also been conducted to demonstrate that the device is reliable in achieving its intended specifications and use.
技术潜在应用场景及目标客户
CLiKX aims to treat hearing loss in children. Otitis Media with Effusion (OME) is the primary cause (c.90%) of hearing loss in children. According to WHO, nearly 2.5 billion people worldwide will be living with some degree of hearing loss by 2050 – that’s 1 in 4 persons. Hearing loss in children caused by OME can be prevented and treated via surgery. Untreated, there can be brain infections, ear drum rupture, delays in hearing, cognition, speech, learning, future socioeconomic limitations and great caregiver burden. In both developed and even more acutely in developing countries, due to the need for GA, high cost of surgery, poor healthcare access/ infrastructure and the low ENT- patient ratio, many do not adopt surgery or get to surgery. Globally, 120 million OME surgeries are required annually but only 18 million currently get to surgery. With CLiKX we can significantly reduce the striking unmet needs.
二、益比盛:世界首款益生菌啤酒
项目情况
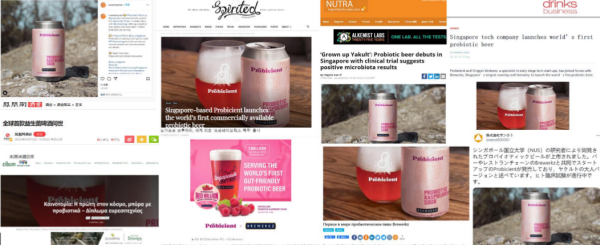
项目通过对菌种的高通量筛选,结合代谢调控技术、微胶囊化技术、定向发酵技术,提高益生菌在复杂(如含醇、酸、抗菌化合物、室温)环境中的活性与稳定性。项目技术创新应用在啤酒行业,精研益生菌啤酒的健康功能性,通过生物活性物质分析(Journal of Agricultural and Food Chemistry 封面文章)与临床试验研究其对饮酒所引发健康风险与疾病的抑制作用。项目依托新加坡国立大学的新加坡初创公司 Probicient,拥有 4 项益生菌啤酒 PCT 专利,具有持续研发能力及丰富渠道资源。一代产品冷藏型益生菌啤酒,上市首销1万升,并入围2022 年“亚洲营养成分大奖”三甲。
项目于 2022 年底落地苏州,转战发展国内市场。团队负责人陈代博士,精研微生物发酵13年,5年益生菌啤酒研发产业化经验。CEO Pung Yan Kii Theodore, 酒类行业资深经理人,创办新式娘惹酒吧,年营业额超千万;高级顾问Liu Shao Quan博士,新国大副教授,从事微生物发酵代谢研究33年,发表SCI文章260余篇。
目前,公司研发的二代产品常温型益生菌啤酒达到可量产阶段,计划明年生产上市。